Crude oil and fossil fuels
and why their characterization is important
Crude oil is one of the most complex mixtures in the world and was generated under elevated temperatures and pressure from organic sediments in the upper earth’s crust over millions of years. It is mainly composed of saturated and unsaturated hydrocarbons, aromatics, as well as a minor portion of polar compounds which contains nitrogen, oxygen or sulphur as heteroatoms and small amounts of metals.
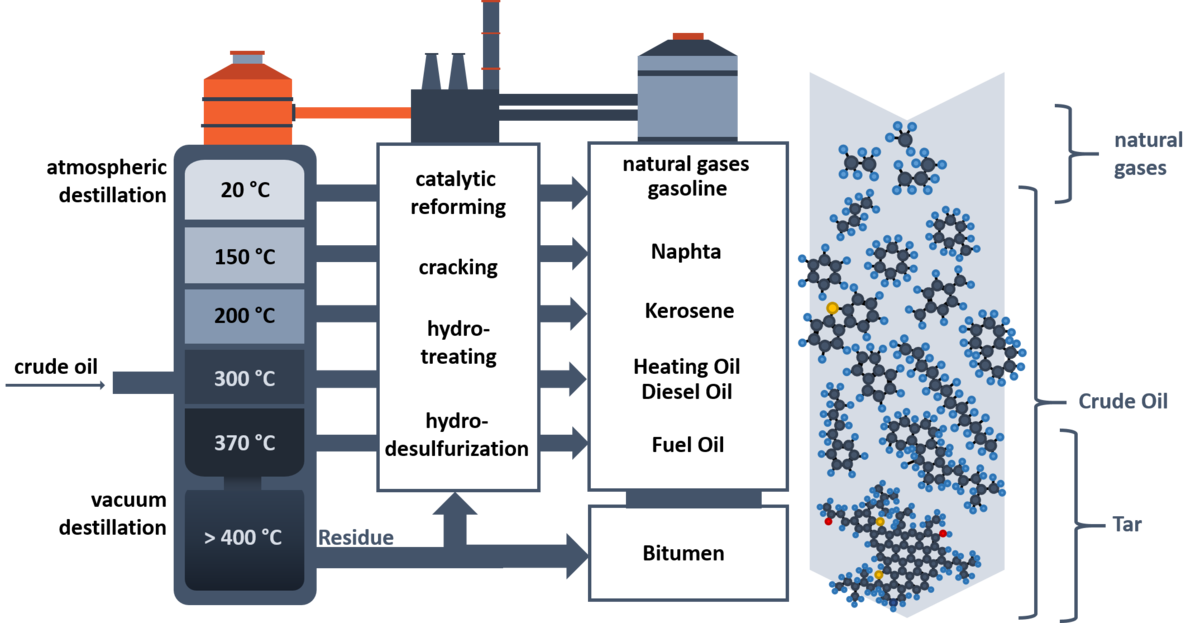
Within the refinery process, the recovered crude oil is fractionated in different distillation cuts by atmospheric pressure distillation followed by vacuum distillation. Lighter distillation cuts are composed of shorter hydrocarbons, whereas heavier distillation cuts contain larger and higher aromatic hydrocarbons with a larger amount of heteroatoms. Consequently, residues of the vacuum distillation contain high aromatic species with a high amount of heteroatoms and metals. With several refinery techniques, such as cracking, hydrotreating and catalytical reforming, the heavier fractions can be transformed into lighter ones. Lighter distillation cuts are used for example as gasoline and kerosene. Middle distillates cover for example diesel ois, marine gas oil (MGO) and heating oils. The distillation residues are used as heavy fuel oil for ships, lubrication oils and bitumens/tars.
Fossil energy sources are needed for the generation of electricity and fuel. Due to the high demand, oil reservoirs containing lighter crude oils get rare and harder accessible reservoirs as well as heavier crude oils gain in importance. The analytics of the physical properties, but also the chemical composition of the crude oil are crucial for an easy production, storage and refinery of the crude oil.